Patient identification
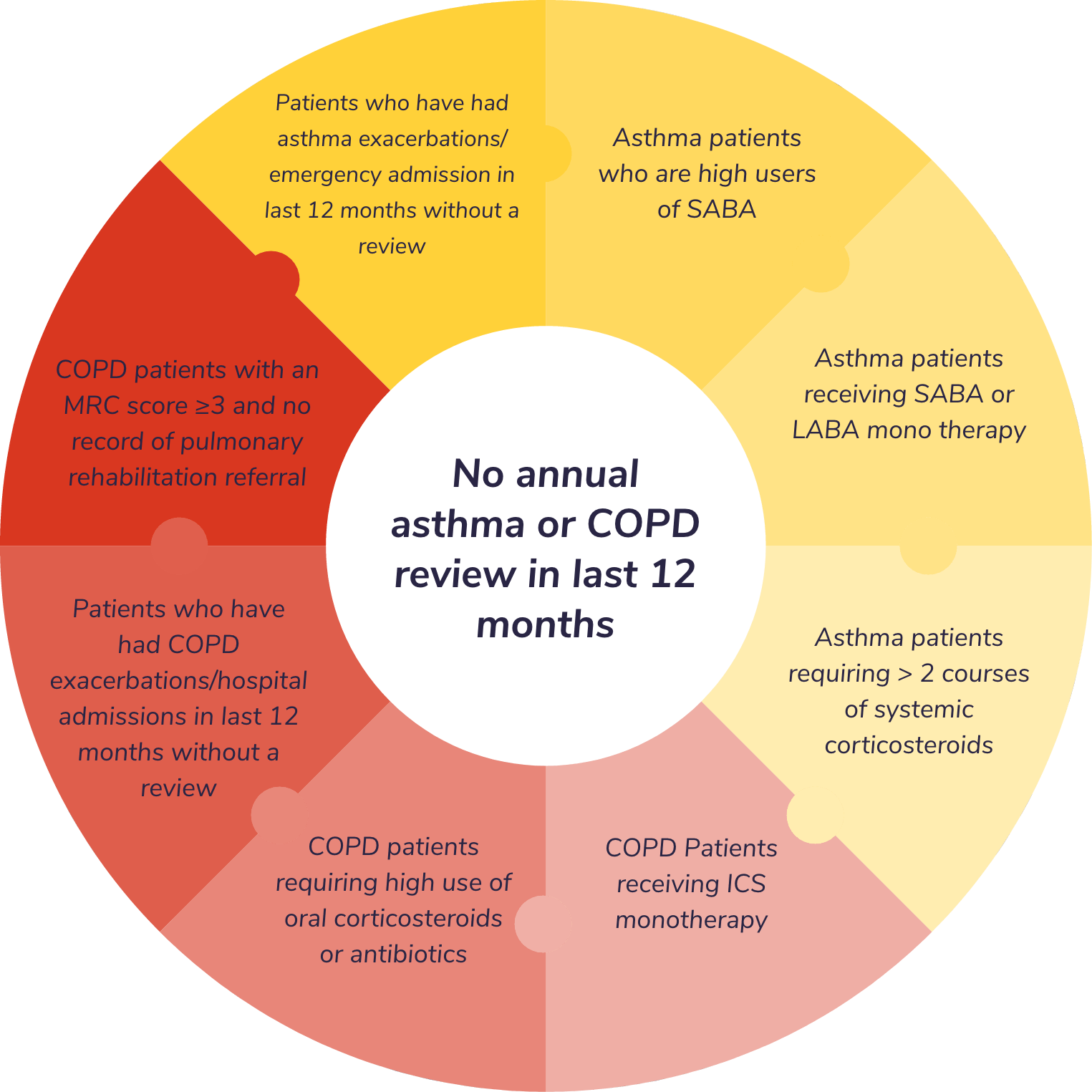
No annual asthma or COPD review in last 12 months
The development of the Toolkit and its delivery was sponsored by GlaxoSmithKline until 13th July 2023.
GlaxoSmithKline was involved in scoping for the Toolkit and reviewed the Toolkit for compliance with the ABPI Code.
Incorporates SNOMED CT coding
Toolkit includes eight distinct protocol alerts and a consolidated search report. All free to download
Both the National Review of Asthma Deaths (1) and National COPD Audit Programme (2) have identified a series of avoidable risk factors for patients living with asthma and COPD. Corresponding quality improvement recommendations to reduce the number of preventable attacks and improve disease control have been made with many of these recommendations echoed in both national policy and guidance issued by organisations such as NICE, BTS/SIGN, NHSE, MHRA, GINA and GOLD (3-12).
of asthma patients who died had been prescribed more than 12 short-acting reliever inhalers in the year before they died
of COPD patients received a prescription for ICS alone (not indicated in COPD)
of asthma patients who died, had no evidence that an asthma review had taken place in general practice in the last year before death
of COPD patients had had two or more exacerbations in the last 12 months
of asthma patients who died had attended a hospital emergency department with asthma at least once in the previous year
of COPD patients did not have an MRC score recorded in the past year. Only 50% of patients with an MRC score of 3-5 had a record of a referral for pulmonary rehabilitation in the past 3 years
Nonetheless, the identification of ‘high risk’ patients for priority review in primary care can often be challenging. The co-ordination of annual reviews for patients with asthma and COPD represents a significant undertaking at a time when resources are under considerable pressure. A proactive and targeted audit can be onerous, and variations in clinical processes and gaps in clinical coding can sometimes result in sub-optimal reporting. Furthermore, limitations and lack of intra-operability associated with GP clinical systems can make the consistent application of best-practice problematic. With these challenges in mind, we have developed the Asthma and COPD Audit.
The Asthma and COPD Audit and Review Toolkit is a simple resource designed to support practitioners in identifying those patients who will most benefit from a review without contributing unduly to practice workload burden.
A search report and protocol alerts to identify asthma and COPD patients at risk of unplanned admissions and/or displaying signs of poor disease control.
Identification criteria consistent with many of the risk factors highlighted by NRAD (1) and National COPD Audit (2).
Facilitates both opportunistic flagging of patients via protocol alerts and systematic audit across practice records using pre-designed search reports. Each search report is designed to facilitate the further prioritisation of at risk patients.
Developed in partnership between the Midlands Practice Pharmacy Network and Prescribing Decision Support Ltd at Keele University.
Available to download and free to use. Compatible with EMIS Web.

No annual asthma or COPD review in last 12 months
With special thanks to the following who provided clinical and technical expertise to support the toolkit’s development: Dr John Burrell, Wolverhampton; Jaz Dhillon, Walsall; Sukhwinder Gill, Walsall; Dr Katherine Hickman, Bradford; Javed Iqbal, Cannock; Jas Johal, Dudley; Alison Webster, Liverpool; Minesh Parbat, Birmingham and Solihull; Bharat Patel, Walsall; Hemant Patel, Wolverhampton; Dr Kay Roy, Hemel Hempstead; Dr Dermot Ryan, Loughborough and Tom Robinson, Dudley.
1. Royal College of Physicians. Why asthma still kills: the National Review of Asthma Deaths (NRAD) Confidential Enquiry report. 2014. 2. Royal College of Physicians. Planning for every breath. National Chronic Obstructive Pulmonary Disease (COPD) Audit Programme: Primary care audit (Wales) 2015–17. National report. 2017. 3. NICE. Asthma: medicines safety priorities. Key therapeutic topic KTT5. Updated March 2019. 4. NHSE. The NHS Long Term Plan. January 2019. 5. Asthma UK. Patient safety failures in asthma care: the scale of unsafe prescribing in the UK. 2015. 6. MHRA. Asthma Long-acting ß2 agonists: use and safety. Guidance. December 2014. 7. Department of Health. Community pharmacy in 2016/17 and beyond. October 2016. 8. British Thoracic Society, Scottish Intercollegiate Guideline Network. SIGN 153 - The British Guideline on the Management of Asthma. 2016. 9. Global Initiative for Asthma. The Global Strategy for Asthma Management and Prevention. 2017. 10. Global Initiative for Asthma. Pocket Guide for Asthma Management and Prevention (for Adults and Children Older than 5 years). 2019. 11. NICE. Chronic obstructive pulmonary disease in adults. Quality standard QS10. February 2016. 12. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2019 report.
Prescribing Decision Support, Centre for Medicines Optimisation, The Hornbeam, Keele University, Keele, Staffordshire, ST5 5BG.
Developed in partnership between the Midlands Practice Pharmacy Network and Prescribing Decision Support Ltd at the Centre for Medicines Optimisation, Keele University. Both parties reserve the right to update and change the Asthma and COPD Audit and Review Toolkit at any time in order to address changes in clinical guidance and best practice, improve functionality and reflect changing user and business needs. Both parties also reserve the right to withdraw the Toolkit if and when its content is out of date and no longer consistent with clinical guidance.
July 2020
© 2020 PDS Ltd